
As the name suggests, they have an affinity for water and are more likely to dissolve in it. In contrast, polar substances or groups are termed hydrophilic or ‘water-loving’.
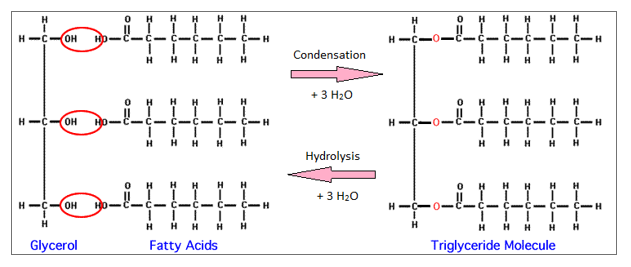

Hydrophobic literally means ‘water-hating’ and generally applies to non-polar substances or chemical groups. The reason for their insolubility in water is that lipids contain large hydrophobic groups. Fats and oils are important in cells as energy storage compounds, while the cell wall itself is constructed from a class of lipids known as phospholipids. The terms ‘fat’ and ‘oil’ simply describe whether a lipid is solid (a fat) or liquid (an oil) at room temperature. Lipids make up the fatty components of living organisms and are the major components of margarine, cooking oil, butter and the white fat associated with meat. What they have in common is that they are all insoluble in polar liquids like water, but soluble in organic (carbon-based) solvents: by this I mean the sort of smelly solvents you tend to find in paints, glues and degreasing agents chloroform is one example. Chemically, lipids are a rather varied group of compounds that include all the substances you might already think of as fats or oils. The cell membrane is constructed from lipids.


 0 kommentar(er)
0 kommentar(er)
